Summary
Aimmune published highly robust Phase 3 (RAMSES, ARTEMIS, and PALISADE) data for AR101. Therefore, the drug is positioned to gain FDA approval for treating peanut allergy by January 2020.
Nonetheless, ICER recently issued a report that questioned the utility of desensitization therapies for peanut allergy.
As opinions rather than facts, the ICER statement is a theory that is used to generate discussion. As such, they do not have any material impact on AR101's approval.
This idea was discussed in more depth with members of my private investing community, Integrated BioSci Investing. Get started today »

The stock market is designed to transfer money from the active to the patient. - Warren Buffet
In bioscience investment, it's crucial to filter out opinions from facts. Despite that you hold a fundamentally sound company, you will face streams of negative market opinions. To stay grounded, it's imperative for you to conduct research due diligence to know when the news is simply a "head fake." This phenomenon is best epitomized by the investment thesis on a food allergy innovator dubbed Aimmune Therapeutics (AIMT).
Though Aimmune delivered extremely robust clinical outcomes for the Phase 3 (RAMSES, ARTEMIS, and PALISADE) trials, its desensitization therapy has been the subject of market negativity. The latest discussion originated from the Institute for Clinical and Economic Review. Notwithstanding, the intelligent Aimmune shareholders are undeterred because such news is simply opinion rather than fact. In this research, I'll present a fundamental analysis of Aimmune while dissecting the negative arguments.
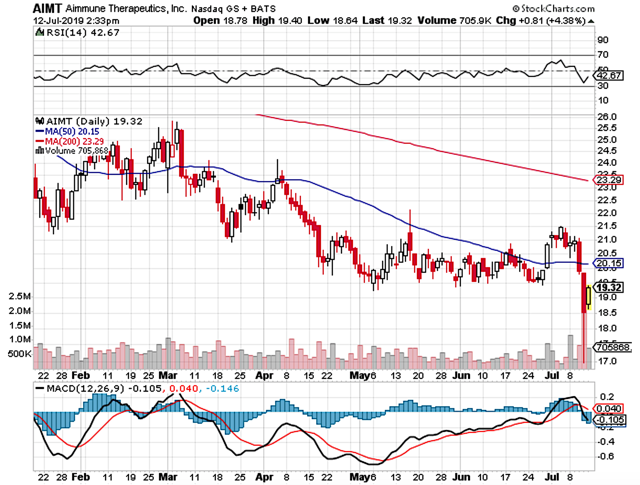 Figure 1: Aimmune chart (Source: StockCharts)
Figure 1: Aimmune chart (Source: StockCharts)About The Company
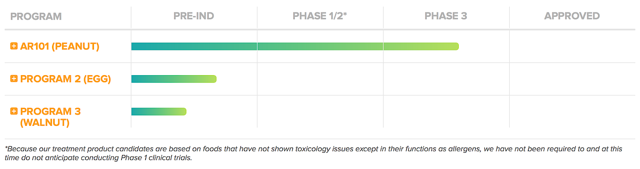
As usual, I'll deliver a brief corporate overview for new investors. If you are familiar with the firm, I suggest that you skip to the subsequent section. Headquartered in Brisbane California, Aimmune Therapeutics is focused on the innovation and commercialization of medicines to serve the food allergy market. As the crown jewel of the pipeline, AR101 is an oral biologic for peanut allergy. Designed by the characterized oral desensitization immunotherapy (i.e. CODIT) platform, AR-101 is a powerful preventative (i.e. prophylactic) therapy. Asides from AR101, Aimmune is expanding its development to serve the unmet needs in other food allergies such as egg and walnut.
CODIT Platform
To appreciate the ramifications of Aimmune's technology, it's important to understand the proprietary CODIT platform. Leveraging oral immunotherapy("OIT"), CODIT is poised to deliver the solution to various food allergies. Like a basketball player that found his range, if one CODIT drug succeeds others will follow suit. I explicated in the prior article,
From the logistics viewpoint, a patient suffering from food allergies like egg, walnut, or peanut would initially visit a specialist doctor (an allergist) for the first dose. Thereafter, maintenance doses can be conveniently administered at home until another follow-up dose is given at the office. After desensitization is achieved, the patient continues with maintenance therapy and avoids allergen exposure. Concurrently, the patient carries an epinephrine auto-injector pen (i.e. epi-pen) as the extra safety step. Investors should realize that CODIT medicines are designed to substantially lower the chances of having a potentially fatal reaction (i.e. anaphylaxis). Yet in the rare case that anaphylaxis occurs, there's always the epi-pen for "rescued" therapy.
 Figure 3: CODIT technology (Source: Aimmune)
Figure 3: CODIT technology (Source: Aimmune)Institute For Clinical And Economic Review
On July 10, 2019, the Institute for Clinical and Economic Review (ICER) issued a report regarding AR101, Viaskin, and other oral immunotherapies (OIT). The aforesaid report featured opinions against the utility of desensitization therapy for peanut allergy. It seems as if the financial market is perturbed yet the poised investors are undeterred. From the corporate front, Aimmune management promptly countered with a defense in the subsequent day. Seeing their eagerness to defend AR101's therapeutic merits for shareholders and patients, I'm quite reassured. As he injected a dose of reality into the situation, the President and CEO (Dr. Jayson Dallas) elucidated,
From the onset, we have believed that ICER’s attempt to assess the value of AR101 was premature, based too heavily on theoretical discussions rather than real-world insight, and omitted valuable patient perspective. Critical data from our Phase 3 trial program continues to emerge, specifically new patient-reported quality-of-life data that were excluded from the analysis. We believe this final report raises more questions than it answers and should be viewed as an early starting point for future conversations—not the final word—about the value of AR101.
I found Dr. Dallas' argument to be logical and valid. With invaluable real-world experience as a bioscience executive, Dr. Dallas is quite pragmatic. Moreover, the Chief has the best access to patients' experience that, in and of itself, is crucial to assessing therapeutic merits. As Dr. Dallas pointed out, ICER is simply "theorizing" for discussion rather than truly analyzing the evidence of efficacy and safety of AR101. Specifically, I did not see ICER discussed ARTEMIS, RAMSES, and PALISADE. That being said, let me walk you through their claims and my counterarguments. As follow, the three arguments ICER made against AR101 utility include the followings:
1) The requirement to continue treatment indefinitely without longer-term evidence of safety or effectiveness; 2) Desensitization does not eliminate the need for caution to avoid inadvertent peanut exposure; 3) The expected yet burdensome increase in the risk of allergic reactions and epinephrine use in the one-year clinical trials for AR101 and Viaskin Peanut.
Regarding the first assertion, the medical reality is that chronic conditions are managed on a lifelong basis. As noble as it is to demand a one time cure, I strongly believe that is impractical. The fact is that most medicines are taken chronically. For instance, patients with heartburn need to take Prilosec over the years while those with arthritis necessitate continual pain med.
As clinical evidence is paramount to innovation progress, let evaluate the key outcomes of the Phase 3 PALISADE trial. Remarkably, 50.3% of AR101-treated patients tolerate a single-highest dose of 1,000mg of peanut protein compared to the meager 2.4% for patients on the sugar pill (i.e. placebo). This signifies that approximately half of all patients taking AR101 would not suffer from anaphylaxis and thereby deters from needing the epi-pen. Instead, if patients do not use AR101 and consumed peanut it's dollars to doughnuts that they will succumb to anaphylaxis. As confirmations to PALISADES results, similar findings were observed in other advanced studies, including RAMSES and ARTEMIS.
In defense against ICER's second argument, I strongly believe that there is nothing wrong with the fact that patients simply need to continue avoiding peanut despite desensitization. Though AR101 is like a bullet-proof vest against peanut allergy, people wearing a bullet-proof vest should avoid getting shot. The added protection from the vest (i.e. AR101) simply minimizes the damage. Asking for 100% bulletproof is an oxymoron. As such, having an excellent medicine does not confer patients the right to be reckless. AR101 provides patients insurance against the deadly anaphylaxis. And, that's the best we can ask from any food allergy drug. Moreover, we should be appreciative of the hopes that AR101 offers to patients.
That aside, AR101 demonstrated the uncanny ability to desensitize peanut allergy in the aforesaid Phase 3 studies. For instance, the stellar drug increased oral tolerated allergen dose by multiple folds. Nevertheless, I highly doubt there will ever be any cure for any food allergy. Due to the working of immune memory, no molecule can induce the immune system to "unlearn" allergen memory. As a stone engraving can't be erased, once the immune memory is developed it can be only weakened but not unlearned. Desensitization and tolerance are the most realistic goals. After all, managing a food allergy isn't the same as treating a simple bacterial infection where a cure is demanded.
Since AR101 improved tolerance and desensitization, it ultimately decreases allergic reaction and anaphylaxis which altogether lowers the need for an epi-pen. Now, what if the patient forgets the epi-pen? In that case, AR101 would still decrease their chances of dying from anaphylaxis. Else, you can bet that the patient will perish if they forgot their epi-pen and not taking AR101.
In my view, if ICER talks to patients I'm certain they'll uncover that having a peace of mind is invaluable to the patient's quality of life. A philosopher once said that the anticipation of fear is worse than the fear itself. Therefore, it must be highly bothersome for patients to ruminate that one day they might forget their epi-pen and encounter anaphylaxis. Moreover, there is no guarantee that all the food that they consume are peanut-free. Even a trace amount of peanut allergen can induce anaphylaxis. Again, taking into account the patient's experience and clinical data is paramount for innovation progress. While there is nothing wrong with a discussion by ICER, I strongly believe that the FDA's decision will be guided by data and patients' experience.
For ICER's third claim, I believe that it fails to appreciate the appropriate clinical context. Early on in the course of desensitization treatment, patients are likely to experience increase allergic reaction. After all, the immune system is "getting used" to AR101. This phenomenon is similar to when people starting to exercise but they are not used to lifting weights or sprinting. As they hit the gym, their muscles will experience soreness the next day. A week into their exercise routine, they should be comfortable with the workout regimen without feeling painful. As health benefits accumulate over the years, the longer patients work out the more well-being they'll achieve.
Desensitization works quite similar to the aforesaid phenomenon. Be that as it may, the desensitization process occurs gradually over several months. As proof in the pudding, patients can tolerate a much higher dosage of peanut allergen after one year of AR101 therapy completion. Though there is a higher incidence of allergic reaction and epi-pen usage in the first year, it'll lead to substantially less reaction thereafter. After all, no pain no gain.
Amid the strong data and heightened demand for treating food allergy, denying AR1010 is a disservice to countless patients and a deterrence to innovation. As such, I believe that AR101 will most likely to gain approval in the U.S. and Europe. In all probability, AR101 will be approved in the U.S. by January 2020. Therefore, I ascribed the 65% (more than favorable) chances of success. Notwithstanding, I anticipated a "focused launch" to commence promptly thereafter.
Financials Assessment
Just as you would get an annual physical for your well-being, it's important to check up on the financial health of your stock. For instance, your health is affected by "blood flow" as your stock's viability is dependent on the "cash flow." With that in mind, I'll analyze the 1Q2019 earnings report for the period that concluded on March 31. Since I already discussed the financial results in detail in the previous article, I'll briefly mention the most salient points.
As follow, Aimmune registered $54.4M ($0.87 per share) net loss compared to $49.5M ($0.92 per share) decline for the same period a year prior. On a per-share basis, this represents an 8.0% earnings improvement. Additionally, the research and development (R&D) expenses registered at $31.3M and thereby signifies a 6.2% decrease from $33.4M for the same year-over-year (YOY) comparison.
Regarding the balance sheet, there was $296.3M in cash and equivalent, thus underlying a 2.5% decrease from $303.9M last year. Based on the $55.0M quarterly OpEx, there should be adequate capital to fund operations for approximately three more years. Excessive offerings can dilute the share count so let's analyze the dilution rate. As the shares outstanding increased from 53.5M to 62.0M for Aimmune, my rough arithmetics yield the 15.9% dilution. At this pace, Aimmune easily cleared my 30% dilution cutoff for a profitable investment. Hence, this company is most definitely not a serial diluter.
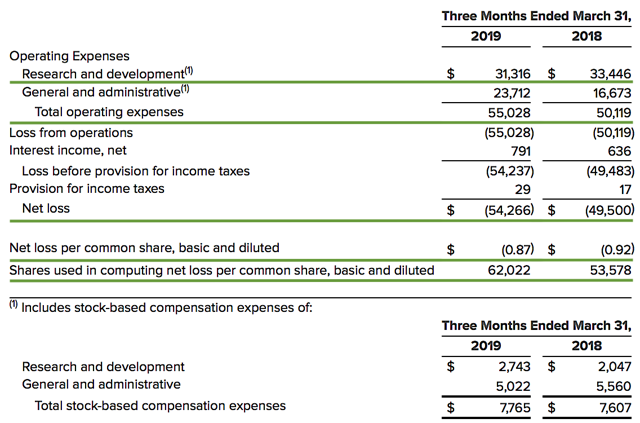 Figure 4: Key financial metrics (Source: Aimmune)
Figure 4: Key financial metrics (Source: Aimmune)Potential Risks
Since investment research is an imperfect science, there are always risks associated with your stock regardless of its fundamental strengths. More importantly, the risks are "growth-cycle dependent." At this point in its life cycle, the main concern for Aimmune is whether AR101 can gain FDA approval by January 2020. Regardless of the prudent launch preparation, AR101 could fail in commercialization because it's difficult for a small company to succeed without a launch partner. Be that as it may, Aimmune is highly strategic in its launch strategy.
With proper preparation and planning, the chances of success are magnified. In light of the heightened demand for long-term prophylaxis for peanut allergy, there is a strong case for robust sales traction. In spite of the negative reports against OIT, I believe they have no materials risk because they are simply opinions.
Final Remarks
In all, I maintain my buy recommendation on Aimmune with the four out of five stars rating. As a master CODIT innovator, Aimmune can synthesize the "silver bullets" for various food allergies. Since there is no available long-term food allergy prophylactic, the demand for CODIT drugs is extremely strong. Already demonstrated stellar Phase 3 data in three high-quality trials, the first CODIT drug (AR101) is poised for approval in 1Q2020 as a treatment for peanut allergy. Amid the blockbuster food allergy market, AR101 is positioned to deliver significant sales. That aside, if the combination trial of AR101 and dupilumab of Regeneron bears fruit Aimmune is most likely to be acquired by Regeneron.
As usual, the choice to buy, sell, or hold is ultimately yours to make. In my view, it's prudent to keep Aimmune. And, it's important not to be swayed by negative opinions. Let the data and purity of science be your guide. Theoretical discussions are talks. At the end of the day, the data and patient experience are paramount to the FDA.
Thanks for reading! Please hit the orange "Follow" button on top for updates.
Dr. Tran's analyses are the best in the biotech sphere, well worth the price of subscription.
Very professional, extremely knowledgeable, and very honest… I would highly recommend this service and his stock picks have been very profitable.
Simply put, this is worth every penny. Just earlier today, one of the companies recommended by Dr. Tran got acquired for a nice 50% premium.
As I reserve higher market intelligence and exclusive features for IBI members, I invite you to take my temporary offer of 2 weeks FREE TRIAL.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it. I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: As a medical doctor/market expert, Dr. Tran is not a registered investment advisor. Despite that we strive to provide the most accurate information, we neither guarantee the accuracy nor timeliness. Past performance does NOT guarantee future results. We reserve the right to make any investment decision for ourselves and our affiliates pertaining to any security without notification except where it is required by law. We are also NOT responsible for the actions of our affiliates. The thesis that we presented may change anytime due to the changing nature of information itself. Investment in stocks and options can result in a loss of capital. The information presented should NOT be construed as recommendations to buy or sell any form of security. Our articles are best utilized as educational and informational materials to assist investors in your own due diligence process. That said, you are expected to perform your own due diligence and take responsibility for your actions. You should also consult with your own financial advisor for specific guidance, as financial circumstances are individualized.